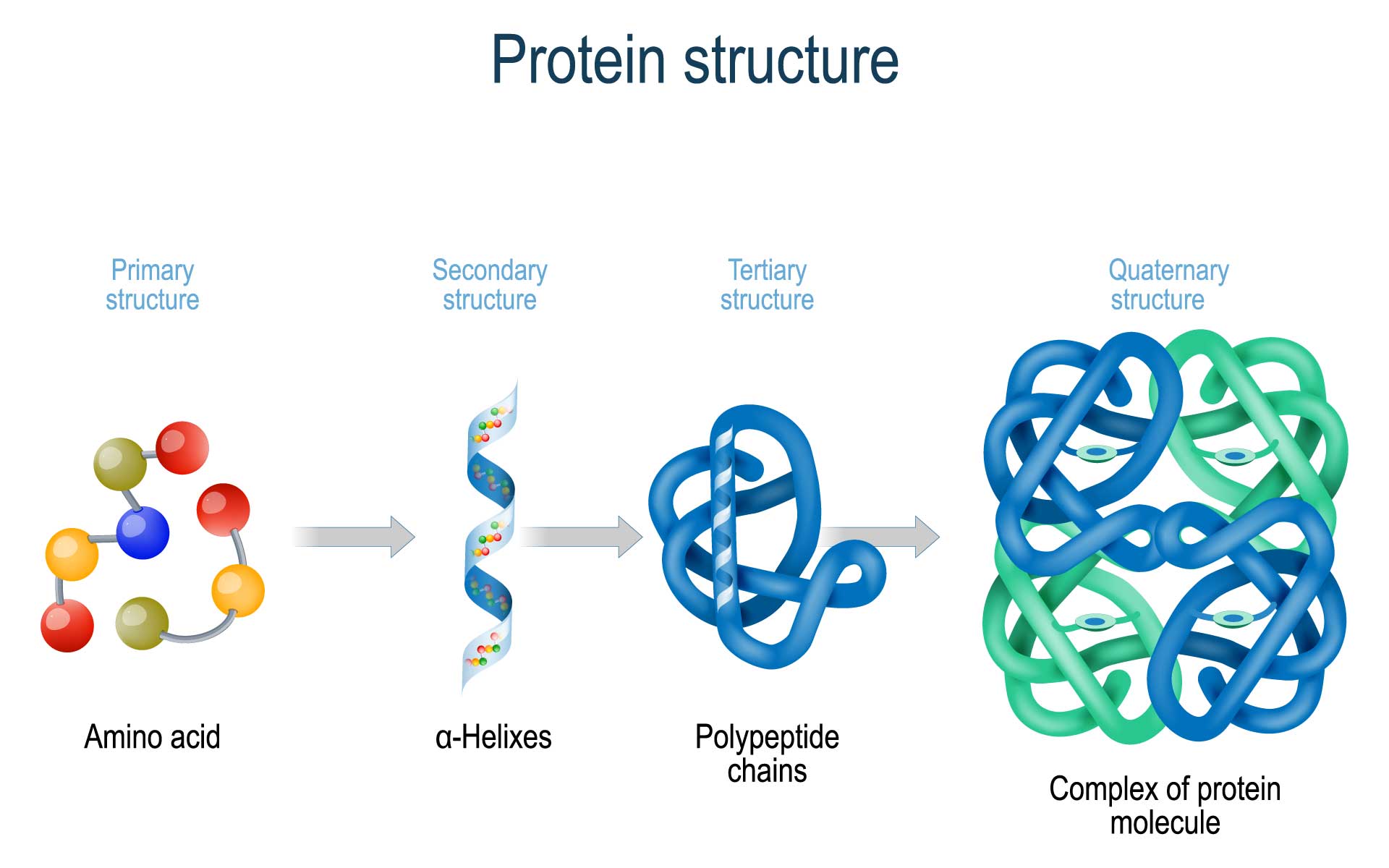
Chaperone Proteins Definition Biology . often the energy of atp is used for supporting conformational transitions in the chaperone proteins, which regulate. we define a molecular chaperone as any protein that interacts with, stabilizes or helps another protein to. initially named heat shock proteins (hsps), it is now known that upregulation of chaperone proteins is a. They protect proteins when they are in the process of folding, shielding them. chaperones are proteins that guide proteins along the proper pathways for folding. chaperones are a group of proteins that have functional similarity and assist in protein folding. understanding chaperone function at the atomic level, and in particular its mode of interaction with client proteins, is crucial to. chaperone proteins, or molecular chaperones, are proteins that assist others to fold properly during or after synthesis, to.
from martinswellness.com
we define a molecular chaperone as any protein that interacts with, stabilizes or helps another protein to. chaperone proteins, or molecular chaperones, are proteins that assist others to fold properly during or after synthesis, to. They protect proteins when they are in the process of folding, shielding them. chaperones are a group of proteins that have functional similarity and assist in protein folding. often the energy of atp is used for supporting conformational transitions in the chaperone proteins, which regulate. understanding chaperone function at the atomic level, and in particular its mode of interaction with client proteins, is crucial to. initially named heat shock proteins (hsps), it is now known that upregulation of chaperone proteins is a. chaperones are proteins that guide proteins along the proper pathways for folding.
Complete Protein or Complete Myth?
Chaperone Proteins Definition Biology we define a molecular chaperone as any protein that interacts with, stabilizes or helps another protein to. understanding chaperone function at the atomic level, and in particular its mode of interaction with client proteins, is crucial to. chaperones are a group of proteins that have functional similarity and assist in protein folding. initially named heat shock proteins (hsps), it is now known that upregulation of chaperone proteins is a. chaperone proteins, or molecular chaperones, are proteins that assist others to fold properly during or after synthesis, to. chaperones are proteins that guide proteins along the proper pathways for folding. They protect proteins when they are in the process of folding, shielding them. we define a molecular chaperone as any protein that interacts with, stabilizes or helps another protein to. often the energy of atp is used for supporting conformational transitions in the chaperone proteins, which regulate.
From batmanaspen.weebly.com
Define chaperone protein batmanaspen Chaperone Proteins Definition Biology They protect proteins when they are in the process of folding, shielding them. chaperone proteins, or molecular chaperones, are proteins that assist others to fold properly during or after synthesis, to. chaperones are a group of proteins that have functional similarity and assist in protein folding. initially named heat shock proteins (hsps), it is now known that. Chaperone Proteins Definition Biology.
From www.cosmeticsdesign.com
Supervisome™ EPH & Chaperone Proteins The Key To HealthyLooking Skin Chaperone Proteins Definition Biology often the energy of atp is used for supporting conformational transitions in the chaperone proteins, which regulate. chaperones are proteins that guide proteins along the proper pathways for folding. chaperone proteins, or molecular chaperones, are proteins that assist others to fold properly during or after synthesis, to. They protect proteins when they are in the process of. Chaperone Proteins Definition Biology.
From www.chegg.com
Solved (a) (b) (c) Molecular Chaperones • Proteins that help Chaperone Proteins Definition Biology chaperone proteins, or molecular chaperones, are proteins that assist others to fold properly during or after synthesis, to. chaperones are a group of proteins that have functional similarity and assist in protein folding. They protect proteins when they are in the process of folding, shielding them. chaperones are proteins that guide proteins along the proper pathways for. Chaperone Proteins Definition Biology.
From www.sciencephoto.com
Bacterial proteinchaperone complex Stock Image C015/0093 Science Chaperone Proteins Definition Biology initially named heat shock proteins (hsps), it is now known that upregulation of chaperone proteins is a. we define a molecular chaperone as any protein that interacts with, stabilizes or helps another protein to. They protect proteins when they are in the process of folding, shielding them. chaperones are a group of proteins that have functional similarity. Chaperone Proteins Definition Biology.
From study.com
Polypeptide Chain Definition & Structure Lesson Chaperone Proteins Definition Biology often the energy of atp is used for supporting conformational transitions in the chaperone proteins, which regulate. chaperones are a group of proteins that have functional similarity and assist in protein folding. initially named heat shock proteins (hsps), it is now known that upregulation of chaperone proteins is a. we define a molecular chaperone as any. Chaperone Proteins Definition Biology.
From pubs.acs.org
Artificial Molecular Chaperone Systems for Proteins, Nucleic Acids, and Chaperone Proteins Definition Biology we define a molecular chaperone as any protein that interacts with, stabilizes or helps another protein to. They protect proteins when they are in the process of folding, shielding them. initially named heat shock proteins (hsps), it is now known that upregulation of chaperone proteins is a. often the energy of atp is used for supporting conformational. Chaperone Proteins Definition Biology.
From dikipromo.weebly.com
Define chaperone protein dikipromo Chaperone Proteins Definition Biology chaperones are proteins that guide proteins along the proper pathways for folding. understanding chaperone function at the atomic level, and in particular its mode of interaction with client proteins, is crucial to. They protect proteins when they are in the process of folding, shielding them. chaperone proteins, or molecular chaperones, are proteins that assist others to fold. Chaperone Proteins Definition Biology.
From www.slideserve.com
PPT Protein Localization PowerPoint Presentation, free download ID Chaperone Proteins Definition Biology understanding chaperone function at the atomic level, and in particular its mode of interaction with client proteins, is crucial to. chaperone proteins, or molecular chaperones, are proteins that assist others to fold properly during or after synthesis, to. chaperones are a group of proteins that have functional similarity and assist in protein folding. often the energy. Chaperone Proteins Definition Biology.
From www.pnas.org
The endoplasmic reticulum chaperone BiP is a closureaccelerating Chaperone Proteins Definition Biology chaperone proteins, or molecular chaperones, are proteins that assist others to fold properly during or after synthesis, to. chaperones are proteins that guide proteins along the proper pathways for folding. They protect proteins when they are in the process of folding, shielding them. initially named heat shock proteins (hsps), it is now known that upregulation of chaperone. Chaperone Proteins Definition Biology.
From www.takarabio.com
Chaperone plasmid set Chaperone Proteins Definition Biology understanding chaperone function at the atomic level, and in particular its mode of interaction with client proteins, is crucial to. initially named heat shock proteins (hsps), it is now known that upregulation of chaperone proteins is a. They protect proteins when they are in the process of folding, shielding them. we define a molecular chaperone as any. Chaperone Proteins Definition Biology.
From www.physicallensonthecell.org
Chaperoneaided Protein Folding Physical Lens on the Cell Chaperone Proteins Definition Biology understanding chaperone function at the atomic level, and in particular its mode of interaction with client proteins, is crucial to. often the energy of atp is used for supporting conformational transitions in the chaperone proteins, which regulate. They protect proteins when they are in the process of folding, shielding them. chaperones are a group of proteins that. Chaperone Proteins Definition Biology.
From www.frontiersin.org
Frontiers Role of ChaperoneMediated Autophagy in Ageing Biology and Chaperone Proteins Definition Biology initially named heat shock proteins (hsps), it is now known that upregulation of chaperone proteins is a. often the energy of atp is used for supporting conformational transitions in the chaperone proteins, which regulate. chaperone proteins, or molecular chaperones, are proteins that assist others to fold properly during or after synthesis, to. They protect proteins when they. Chaperone Proteins Definition Biology.
From www.writework.com
A general overview of chaperone proteins, and the purpose, function and Chaperone Proteins Definition Biology chaperones are proteins that guide proteins along the proper pathways for folding. They protect proteins when they are in the process of folding, shielding them. we define a molecular chaperone as any protein that interacts with, stabilizes or helps another protein to. chaperone proteins, or molecular chaperones, are proteins that assist others to fold properly during or. Chaperone Proteins Definition Biology.
From jonlieffmd.com
Protein Folding in the Neuron and the Mind Jon Lieff, M.D. Chaperone Proteins Definition Biology chaperones are proteins that guide proteins along the proper pathways for folding. we define a molecular chaperone as any protein that interacts with, stabilizes or helps another protein to. They protect proteins when they are in the process of folding, shielding them. chaperones are a group of proteins that have functional similarity and assist in protein folding.. Chaperone Proteins Definition Biology.
From cosmosmagazine.com
A protein chaperone Chaperone Proteins Definition Biology chaperone proteins, or molecular chaperones, are proteins that assist others to fold properly during or after synthesis, to. understanding chaperone function at the atomic level, and in particular its mode of interaction with client proteins, is crucial to. chaperones are a group of proteins that have functional similarity and assist in protein folding. chaperones are proteins. Chaperone Proteins Definition Biology.
From www.semanticscholar.org
Figure 1 from The function of chaperone proteins in the assemblage of Chaperone Proteins Definition Biology chaperones are a group of proteins that have functional similarity and assist in protein folding. initially named heat shock proteins (hsps), it is now known that upregulation of chaperone proteins is a. understanding chaperone function at the atomic level, and in particular its mode of interaction with client proteins, is crucial to. often the energy of. Chaperone Proteins Definition Biology.
From slideplayer.com
Macromolecules Part 2 Unit 1 Chapter ppt download Chaperone Proteins Definition Biology often the energy of atp is used for supporting conformational transitions in the chaperone proteins, which regulate. chaperones are a group of proteins that have functional similarity and assist in protein folding. chaperone proteins, or molecular chaperones, are proteins that assist others to fold properly during or after synthesis, to. understanding chaperone function at the atomic. Chaperone Proteins Definition Biology.
From www.youtube.com
chaperone protein folding chaperona HSP molecular biology biología Chaperone Proteins Definition Biology understanding chaperone function at the atomic level, and in particular its mode of interaction with client proteins, is crucial to. chaperones are proteins that guide proteins along the proper pathways for folding. initially named heat shock proteins (hsps), it is now known that upregulation of chaperone proteins is a. chaperones are a group of proteins that. Chaperone Proteins Definition Biology.